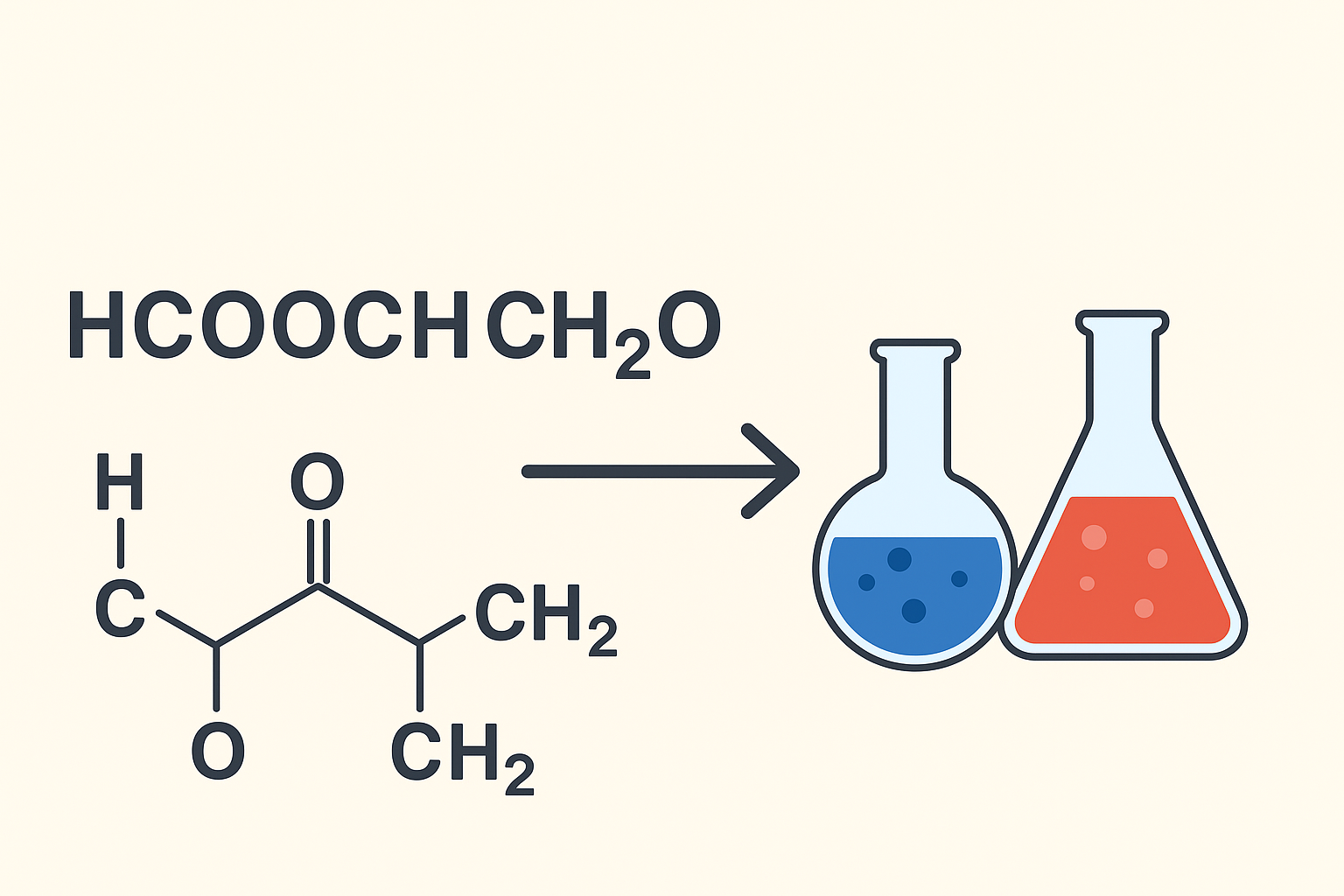
Chemistry is full of fascinating formulas that describe the behavior of different compounds. One such formula is hcooch ch2 h2o. While it may look complex at first, breaking it down makes it easier to understand. For students in school, especially around the 9th-grade level, learning these formulas helps build a strong foundation in science. In this article, we will explore what this formula means, how it is structured, and why it is important. Additionally, we will look at its role in chemical reactions and its uses in daily life.
By the end, you will not only understand the chemical but also gain confidence in reading and analyzing chemical equations.
What Does hcooch ch2 h2o Mean?
The formula hcooch ch2 h2o represents a combination of organic molecules with water. At first glance, it looks unusual because it is written in a way that blends functional groups together. Breaking it down is the best way to understand it.
-
HCOOCH suggests the presence of an ester group. Esters are organic compounds formed when an acid reacts with an alcohol. They often have pleasant smells and are widely used in perfumes and flavorings.
-
CH2 indicates the presence of a methylene group, which acts as a connecting unit between larger molecules.
-
H2O stands for water, which is a universal solvent and one of the most important compounds in chemistry and life.
So, this formula highlights a structure that connects esters, hydrocarbons, and water, showing that it may represent a reaction or interaction between these components.
Understanding the Components
To fully understand hcooch ch2 h2o, let’s break down its parts into smaller pieces.
Esters (HCOOCH)
Esters are organic molecules derived from carboxylic acids and alcohols. They are represented by the general formula RCOOR’. Esters are widely known for their fruity smell. For example, the smell of bananas and pineapples comes from ester compounds.
Hydrocarbon Group (CH2)
The CH2 group acts as a building block in organic chemistry. It links different molecules and allows the formation of long carbon chains. Hydrocarbons like this make up fuels, plastics, and countless synthetic materials.
Water (H2O)
Water is essential for nearly all chemical reactions, especially in biological systems. It can act as a solvent, medium for reactions, or even a reactant. When added to esters, water can break them down in a process called hydrolysis.
The Role of hcooch ch2 h2o in Reactions
Chemistry is often about how compounds react with one another. The formula hcooch ch2 h2o is most likely related to reactions involving esters and water.
Hydrolysis Reaction
When esters are mixed with water under acidic or basic conditions, they break down into an alcohol and an acid. This reaction is called hydrolysis. For example:
-
Ester + Water → Alcohol + Acid
This process is important in digestion because fats (which are esters) break down into glycerol and fatty acids when acted upon by water and enzymes.
Condensation Reaction
On the other hand, alcohols and acids react to form esters and release water as a byproduct. Chemists call this a condensation reaction. Therefore, hcooch ch2 h2o might also represent a situation where ester formation releases water.
Real-Life Applications of hcooch ch2 h2o
Chemistry formulas like this are not just classroom topics. They appear in daily life in many forms.
In Flavors and Fragrances
Esters (HCOOCH type compounds) are responsible for many fruity flavors and sweet aromas. They are used in perfumes, candles, and artificial flavorings in food.
In Medicines
Ester-based compounds play a role in drug design. Many medicines rely on ester bonds to release active ingredients in the body at the right time.
In Plastics and Fibers
Hydrocarbons like CH2 groups are the backbone of synthetic polymers such as polyethylene and polyester. These materials are found in clothing, packaging, and countless products.
In Biology
Water (H2O) is vital for all life. In combination with organic molecules like esters, it supports metabolic processes such as digestion and energy release.
Properties of hcooch ch2 h2o Related Compounds
When examining a formula, understanding its physical and chemical properties makes it more meaningful.
Physical Properties
-
Esters are often liquids with pleasant smells.
-
Hydrocarbons like CH2 are usually colorless and form chains that can be solid, liquid, or gas depending on size.
-
Water is colorless, tasteless, and odorless.
Chemical Properties
-
Esters react with water in hydrolysis.
-
Hydrocarbons can burn in oxygen to produce carbon dioxide and water.
-
Water acts as a solvent, medium, or reactant in many reactions.
Why Understanding hcooch ch2 h2o Matters for Students
Learning chemistry may sometimes feel abstract, but formulas like hcooch ch2 h2o make the subject practical and real.
-
It connects classroom knowledge with real-world applications.
-
It improves problem-solving skills by encouraging students to analyze chemical structures.
-
It builds a strong foundation for future studies in biology, medicine, and environmental science.
-
It develops appreciation for the chemical basis of everyday life.
Conclusion
The formula hcooch ch2 h2o might look confusing at first, but breaking it down makes it understandable. It represents a combination of esters, hydrocarbons, and water, which together play important roles in reactions like hydrolysis and condensation. Beyond the classroom, you can find compounds related to this formula in perfumes, medicines, plastics, and even biological processes.
For 9th-grade students and beyond, learning about such formulas builds confidence in chemistry and shows how science connects deeply to daily life