Chemistry often feels complex, but it becomes easier once you understand its building blocks. Among these, the ionic bond plays an important role in explaining how atoms come together to form matter. Without ionic bonds, many everyday substances—like salt—would not exist. In this guide, you will learn what ionic bonds are, how they form, their properties, and why they matter in the world around us.
What Is an ionic bond
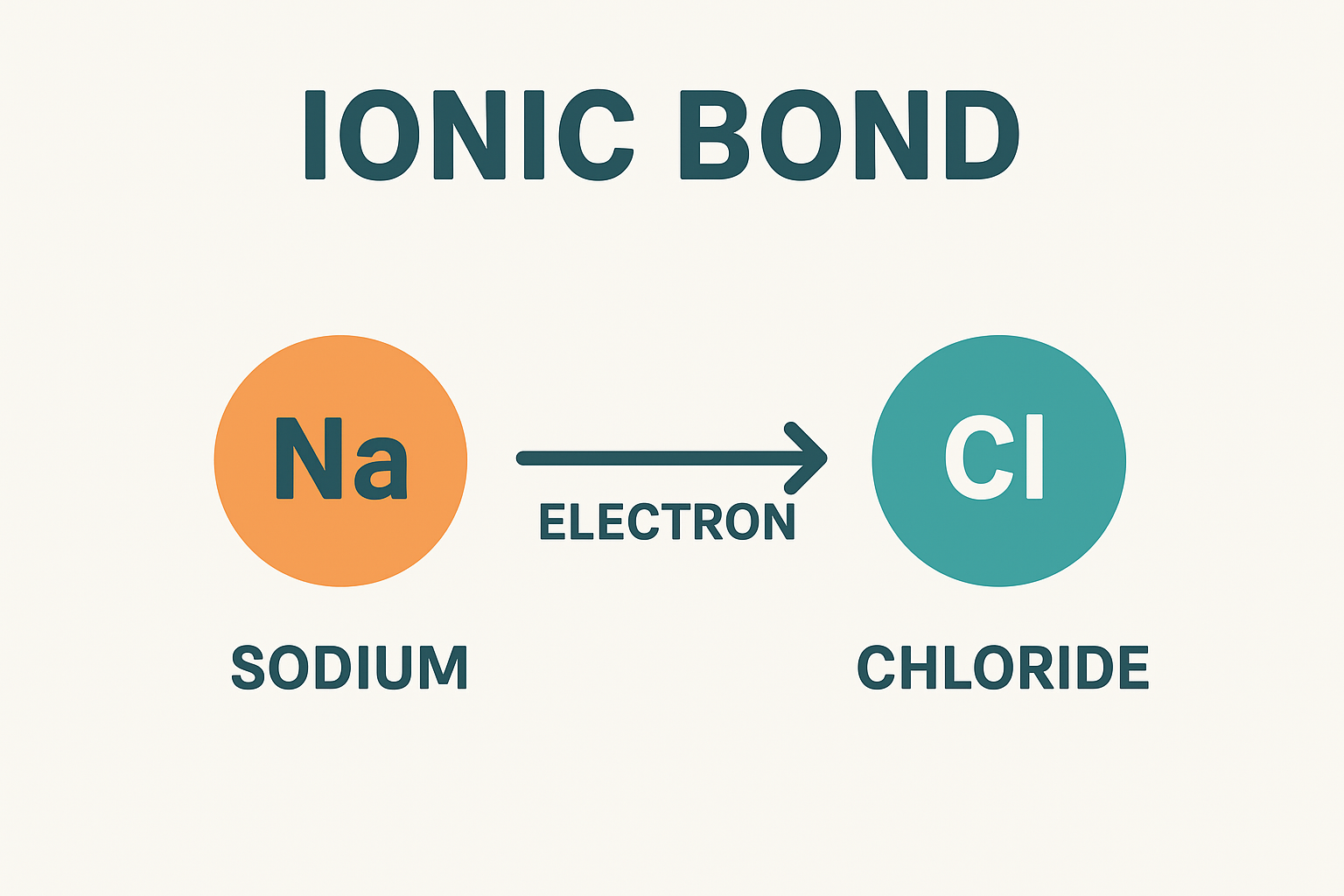
An ionic bond is a type of chemical bond formed when one atom transfers electrons to another. This process creates charged particles called ions. One becomes positively charged (cation), and the other becomes negatively charged (anion). Their opposite charges pull them together, forming a stable compound.
The Basics of Atomic Structure
To understand ionic bonds, we must first look at atoms. Atoms are made of protons, neutrons, and electrons. Protons and neutrons sit in the nucleus, while electrons orbit in shells. Atoms always aim for stability, which usually means having a full outer shell of electrons.
How an ionic bond Forms
An ionic bond forms when one atom gives away an electron while another atom accepts it. For example, sodium has one electron in its outer shell, while chlorine needs one to complete its shell. Sodium gives its electron to chlorine, creating sodium chloride—common table salt.
Role of Electronegativity
Electronegativity is the ability of an atom to attract electrons. Ionic bonds occur when the difference in electronegativity between two atoms is large. Metals like sodium easily lose electrons, while non-metals like chlorine readily gain them.
Properties of ionic bond Compounds
Compounds formed by ionic bonds share several properties:
-
They form crystal lattice structures.
-
They have high melting and boiling points.
-
They conduct electricity when dissolved in water.
-
They are usually solid at room temperature.
These traits make ionic compounds unique compared to other types.
Examples of ionic bond Compounds
Several everyday substances exist because of ionic bonding:
-
Sodium chloride (NaCl) – table salt.
-
Magnesium oxide (MgO) – used in fireproofing.
-
Calcium chloride (CaCl₂) – used for melting ice.
-
Potassium iodide (KI) – used in medicine.
These examples show how common ionic bonds are in daily life.
Comparing ionic bond and Covalent Bond
While ionic bonds involve the transfer of electrons, covalent bonds involve sharing electrons. Ionic bonds usually form between metals and non-metals, while covalent bonds form between non-metals. For example, water (H₂O) has covalent bonds, while salt (NaCl) has ionic bonds.
Importance of ionic bond in Nature
Nature relies on ionic bonds to create stability in compounds. For instance, bones and teeth are strengthened by calcium phosphate, an ionic compound. It also help in nerve signal transmission through sodium and potassium ions.
ionic bond in Everyday Life
>>>>Ionic compounds surround us daily:
-
Salt enhances food flavor.
-
Baking soda aids in cooking and cleaning.
-
Electrolytes like sodium and potassium maintain hydration.
These uses prove that ionic bonds are essential for both survival and comfort.
How ionic bond Compounds Conduct Electricity
Ionic compounds do not conduct electricity in solid form. However, when dissolved in water or melted, their ions move freely. This movement allows the flow of current, making them conductors in these states.
Strength and Stability of ionic bond
The strength of an ionic bond depends on the charge of ions and their size. Higher charges and smaller ion sizes create stronger bonds. For example, magnesium oxide has stronger bonds than sodium chloride because magnesium and oxygen have higher charges.
Lattice Structures in ionic bond Compounds
When ions join, they arrange themselves into a repeating pattern called a lattice. This structure maximizes attraction between opposite charges and minimizes repulsion. As a result, ionic compounds are stable and hard.
ionic bond in Industrial Applications
Industries rely on ionic compounds in many ways:
-
Salt production for food and chemicals.
-
Electrolytes for batteries.
-
Calcium compounds for cement and construction.
-
Chlorine compounds for water treatment.
These applications show how ionic bonds support modern society.
Environmental Impact of ionic bond Compounds
While useful, some ionic compounds can harm the environment. For example, excess salt in soil reduces crop growth, and certain chlorides pollute water. Thus, responsible usage and management are crucial.
Myths About ionic bond
Several myths surround ionic bonds:
-
Myth: Ionic compounds always dissolve in water.
Truth: Some, like barium sulfate, are insoluble. -
Myth: Ionic bonds are weaker than covalent bonds.
Truth: Many ionic bonds are stronger, depending on conditions. -
Myth: All crystals are ionic.
Truth: Some crystals, like quartz, are covalent.
FAQs
Q1: What is an ionic bond in simple words?
It is a bond where one atom gives away an electron, and another takes it, forming ions that attract each other.
Q2: What are the main properties of ionic compounds?
They have high melting points, form crystals, and conduct electricity when melted or dissolved.
Q3: Can ionic bonds form between two metals?
No, they form between metals and non-metals.
Q4: Are ionic bonds stronger than covalent bonds?
Sometimes. It depends on the ions’ charges and sizes.
Q5: Why do ionic compounds conduct electricity in water?
Because their ions move freely, allowing current to pass.
Q6: What is the most common ionic compound?
Table salt (NaCl) is the most common and widely used example.
Conclusion
The ionic bond is a foundation of chemistry. It explains how atoms achieve stability by transferring electrons. From the salt on our tables to the compounds in our bones, ionic bonds influence our daily lives in countless ways. They provide strength, stability, and essential properties that make modern living possible. By understanding ionic bonds, students and learners gain a deeper appreciation of how the smallest particles shape the biggest parts of our world.